Introduction
The electron transport chain and ATP Synthesis is a series of four protein complexes that couple redox reactions, creating an electrochemical gradient that leads to the creation of ATP in a complete system called oxidative phosphorylation. It occurs in the mitochondria in both cellular respiration and photosynthesis. In the first, electrons come from the decomposition of organic molecules and energy is released. In the latter, electrons enter the chain after being excited by light, and the energy released is used to build carbohydrates.
Basics
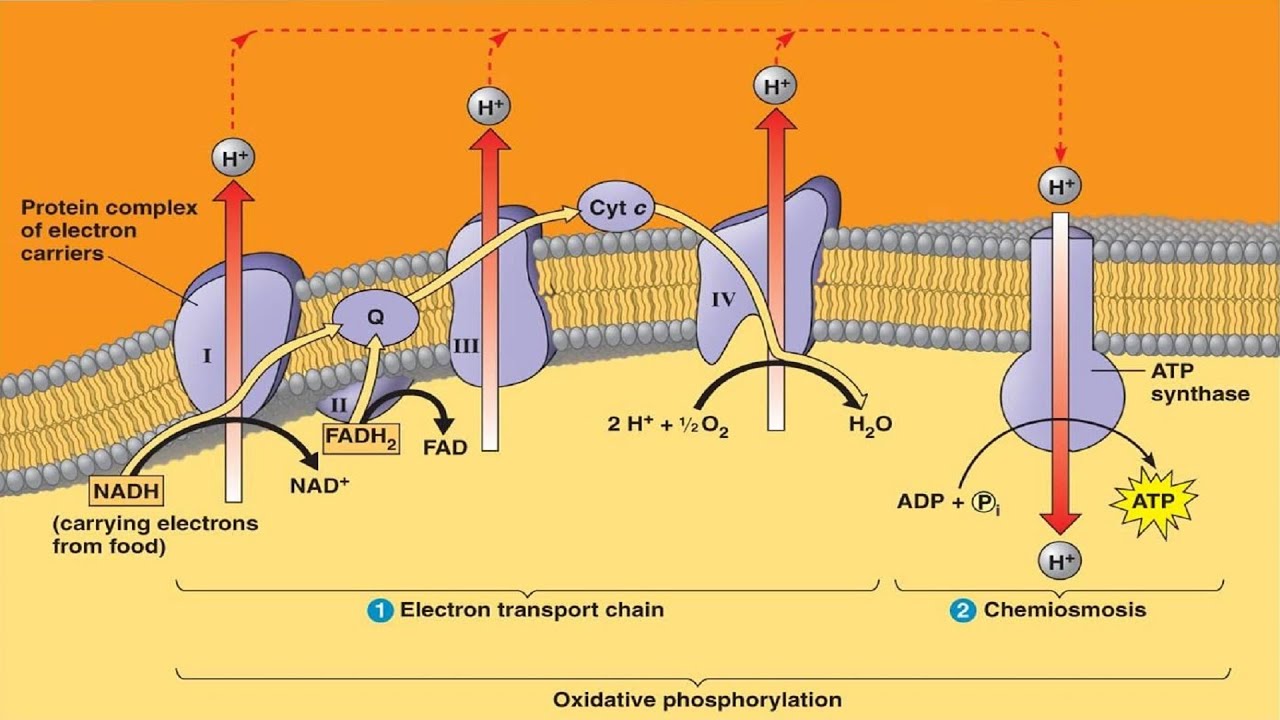
Aerobic cellular respiration is made up of three parts: glycolysis, the citric acid (Krebs) cycle, and oxidative phosphorylation. In glycolysis, glucose is metabolized into two pyruvate molecules, with the production of ATP and nicotinamide adenine dinucleotide (NADH). Each pyruvate is oxidized to acetyl CoA and an additional molecule of NADH and carbon dioxide (CO2). Acetyl CoA is then used in the citric acid cycle, which is a chain of chemical reactions that produce CO2, NADH, flavin adenine dinucleotide (FADH2), and ATP. In the final step, the three NADH and one FADH2 accumulated in the previous steps are used in oxidative phosphorylation to produce water and ATP.
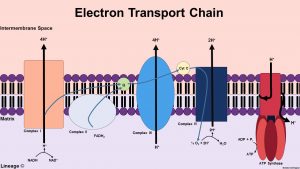
Oxidative phosphorylation has two parts: the electron transport chain (ETC) and chemiosmosis. The ETC is a collection of inner mitochondrial membrane-bound proteins and organic molecules, through which electrons pass in a series of redox reactions and release energy. The released energy forms a proton gradient, which is used in chemiosmosis to produce a large amount of ATP by the protein ATP-synthase.
Photosynthesis is a metabolic process that converts light energy into chemical energy to build sugars. In light-dependent reactions, energy from light and water are used to produce ATP, NADPH, and oxygen (O2). The proton gradient used to make ATP is formed through an electron transport chain. In light-independent reactions, sugar is formed from ATP and NADPH from the previous reactions.
ATP Synthesis
Within cells, energy is provided by the oxidation of “metabolic fuels” such as carbohydrates, lipids, and proteins. It is then used to support energy-dependent processes such as macromolecule synthesis, muscle contraction, active ion transport, or thermogenesis. The oxidation process results in the production of free energy that can be stored in phosphoanhydrin “high-energy bonds” within molecules such as nucleoside diphosphate and nucleoside triphosphate (i.e., adenosine 5′ diphosphate and adenosine 5′ triphosphate, ADP and ATP, respectively). phosphoenolpyruvate, carbamoyl phosphate, 2,3-bisphosphoglycerate and other phosphagens such as phosphoarginine or phosphocreatine.
Among them, ATP is the effective central link (the currency) between energy-producing and energy-demanding processes that actually involve the formation, hydrolysis, or transfer of the terminal phosphate group. In general, the main source of energy for cellular metabolism is glucose, which is catabolized in the following three processes: glycolysis, tricarboxylic acid cycle (TCA or Krebs cycle) and finally oxidative phosphorylation, to produce ATP. In the first process, when glucose is converted to pyruvate, the amount of ATP produced is low.
Pyruvate is subsequently converted to acetyl coenzyme A (acetyl-CoA) which enters the TCA cycle, allowing the production of NADH. Finally, the respiratory chain complexes use NADH to generate a proton gradient across the inner mitochondrial membrane, necessary for the production of large amounts of ATP by mitochondrial ATP synthase. Furthermore, it should be mentioned that acetyl-CoA can also be generated by lipid and protein catabolism.